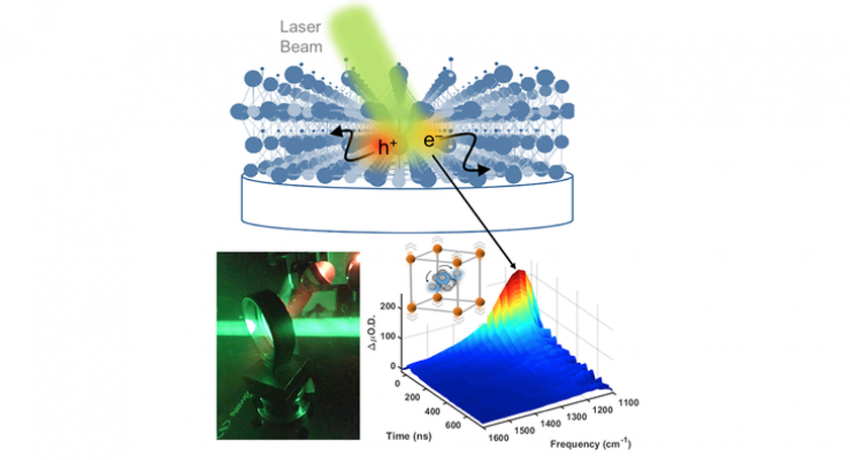
Using ultrafast infrared imaging techniques (bottom left), a team of researchers from Penn State has revealed that the remarkable electronic properties of halide perovskite photovoltaic materials properties arise from large-scale motion of atoms (bottom right) in their crystalline lattice (top). Credit: Asbury Lab, Penn State
New insight into how a certain class of photovoltaic materials allows efficient conversion of sunlight into electricity could position these materials to replace traditional silicon solar cells. A study by researchers at Penn State reveals the unique properties of these inexpensive and quick-to-produce halide perovskites, information that will guide the development of next generation solar cells. The study appears September 27 in the journal Chem.
“Since the development of silicon solar cells, which today can be found on rooftops and roadsides, researchers have sought new types of photovoltaic materials that are easier to process into solar cells,” said John Asbury, associate professor of chemistry at Penn State and senior author of the study. “This is because construction of silicon solar cells is complex and hard to scale-up to the level that would be needed for them to generate even 10 percent of our total demand for electricity.”
Because of these complications, researchers have been searching for less expensive alternatives to silicon solar cells that can be processed more quickly. They are particularly interested in materials that can be processed using a technique called roll-to-roll manufacturing, a technique similar to those used to print newspapers that enables low-cost, high-volume production. Such materials must be processed from solution, like ink printed on a page.
“After forty years of intense research for such materials, nothing has come close to silicon — except an exciting class of materials known as halide perovskites,” said Asbury. “Halide perovskites seem to have a unique tolerance for imperfections in their structures that allow them to efficiently convert sunlight into electricity when other materials with similar imperfections do not.”
What makes halide perovskites so tolerant of imperfections, however, was unknown prior to this study. The researchers used ultrafast infrared imaging technology to investigate how the structure and composition of these materials influence their ability to convert sunlight into electricity.
The researchers determined that halide perovskites have a unique ability to maintain their crystalline structure even while the atoms in their crystals undergo unusually large-scale vibrational motion. All materials experience vibrational motion of their atoms, which is typically suppressed by making the materials’ crystals very hard — like silicon — so that their atoms are rigidly held in place. But, according to the current study, halide perovskites are very soft, which allows their atoms to move around and contributes to their remarkable efficiency.
“What is interesting is that such large-scale atomic motions typically lead to a loss of crystalline structure in other materials, creating imperfections that drain excited state energy,” said Asbury. “But with halide perovskites, researchers can chemically substitute electronically charged atoms in the material to tune the amplitudes of such atomic scale motions. This will allow us to improve the performance and stability of halide perovskite materials.
“Currently, halide perovskites often contain toxic elements like lead and are not yet as stable as they will need to be to replace silicon solar cells,” said Asbury. “The insights from this study will enable us to create rules for designing new halide perovskites using roll-to-roll processing. This will guide the development of next generation perovskite materials that are more stable and that contain less toxic elements such as tin instead of lead.”
In addition to Asbury, the research team at Penn State includes Kyle Munson, Eric Kennehan, and Grayson Doucette. This work was funded by the National Science Foundation.