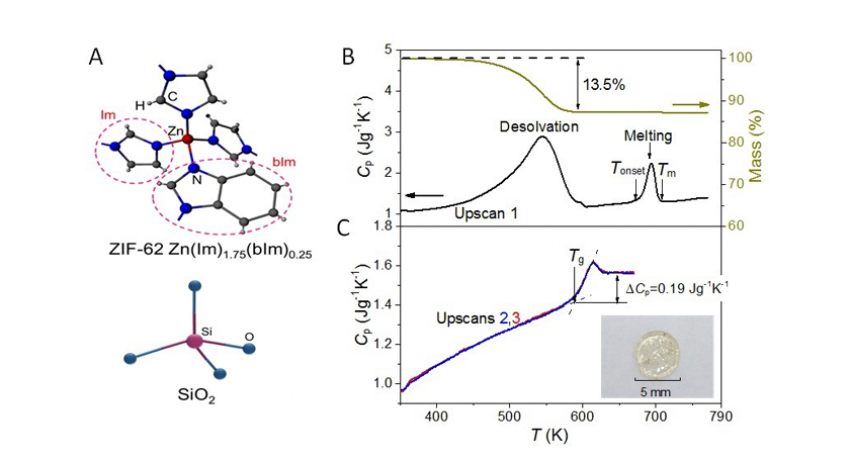
(A) Similarity between tetrahedra in silicate glasses and ZIF-62 Im/bIm networks. (B) Cp and mass loss versus T, heated at 10 K min−1, following desolvation to eventual melting at Tm = 708 K. (C) Cp upscans of ZIF-62 glass quenched from above Tm showing a clear glass transition (Tg = 595 K), yielding Tg/Tm (0.84). Inset: Optical image of a transparent MQ glass. Image: Ang Qiao / Penn State
Lightning and volcanos both produce glass, and humans have been making glass from silicon dioxide since prehistory. Industrialization brought us boron-based glasses, polymer glasses and metallic glasses, but now an international team of researchers has developed a new family of glass based on metals and organic compounds that stacks up to the original silica in glass-forming ability.
Glass-forming ability is the ability of a liquid to avoid crystallization during cooling.
"Glass is a liquid frozen into a solid-like material in noncrystalline form," said John C. Mauro, professor of materials science and engineering, Penn State. "Mechanically it behaves as a solid but it is somewhere between a liquid and a solid."
The key to making glass is to melt the source materials and then somehow manage to cool them so that no crystals form. One way of doing this is by rapid cooling or quenching. This shortens the time available for crystals to form due to the rapid temperature drop.
Basic silica glass has a tetrahedral structure with silicon in the center and four oxygen atoms at the corners. A tetrahedron is a triangular pyramid. Each oxygen attaches to another silicon-centered tetrahedron.
The metal-organic glass the researchers produced substitutes zinc for silicon, but uses two similar but different organic compounds at the corners — imidazolate and benzimidazolate. These organic molecules randomly take the place of the oxygen atoms at the tetrahedron corners.
Read the full news story here:
http://news.psu.edu/story/509446/2018/03/09/research/metal-organic-compounds-...