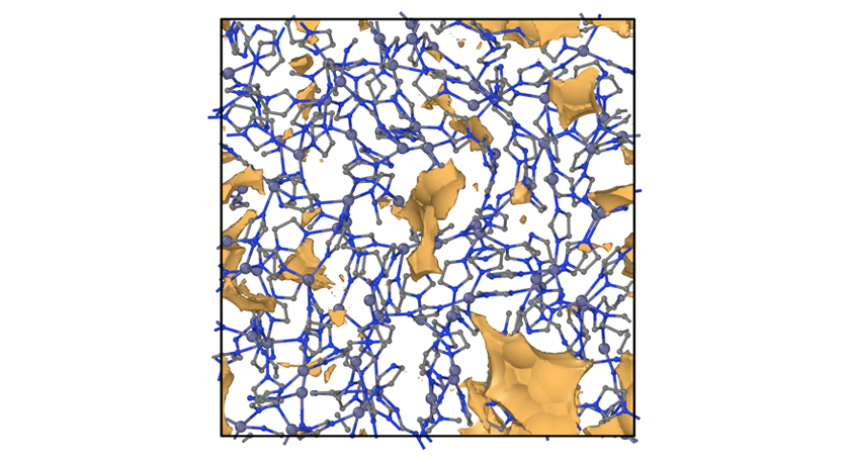
A visualization of the pore structure in ZIF glass from ReaxFF molecular dynamics simulation. Yongjian Yang/Penn State
By Walt Mills
ZIF glasses, a new family of glass, could combine the transparency of silicate glass with the nonbrittle quality of metallic glass, according to researchers at Penn State and Cambridge University, UK.
“We are sure of the transparency,” said John Mauro, professor of materials science and engineering, Penn State. “We’ll have to wait until larger samples can be made to know if it has the amazing ductility of metallic glass, but it looks promising.”
The newest class of glass-forming materials, called ZIFs for zeolitic imidazolate frameworks, has a structure in which metal ions are linked by organic ligands. When heated within a limited range of high temperatures, some ZIF materials will melt and reform into a glassy structure in which the atoms have a disordered structure. Beyond the potential of a transparent and far more bendable glass, some ZIFs contain large numbers of functional pores that can be used for gas storage (metal-organic frameworks have been proposed as cages for hydrogen storage for fuel cell vehicles), catalysis, gas separation or even drug delivery.
“ZIFs are so new that people are just discovering which chemistries form glasses,” Mauro said. “The goal of our group is to accelerate the design of these new glasses through modeling.”
In two recent journal articles, Mauro and colleagues have used different modeling methods to deepen understanding and predict the properties of ZIF glasses. The first modeling method, called ReaxFF, was developed by Penn State professor of mechanical engineering, Adri van Duin, who is co-author of both papers. ReaxFF is a computationally fast and economical method for simulating the melting and reforming of candidate materials.
“It is difficult to simulate these systems because the models are usually developed for either organic systems or inorganic systems, but not both,” said Yongjian Yang, Mauro’s postdoctoral scholar and lead author on both papers. “Plus, unlike ReaxFF, other models don’t allow for the bond breaking and reformation that take place in glass forming.”
Yang added that using ReaxFF cuts the time required to perform simulations to a few hours rather than the several days it would have taken using quantum mechanics methods.
In the most recent paper, published in the Journal of Physical Chemistry Letters, the researchers used another modeling method originally developed for another class of glasses called chalcogenide glasses.
“James Phillips proposed that we can think about glasses in the same way a civil engineer would think about designing a truss structure like in a bridge or the Eiffel Tower,” Mauro said.
Phillips, who was at Bell Labs at the time and is now at Rutgers University, proposed a method to optimize glass based on the degrees of freedom of the atoms compared to the number of rigid bonds to other atoms. When the bonds equal the degrees of freedom -- the ability to move up, down or sideways -- the system is usually in the optimal state to form a stable glass.
Mauro, who was at Corning Incorporated, and Prabhat Gupta, of Ohio State, extended the theory to develop what’s called Temperature Dependent Constraint Theory, which accounts for bond breaking at high temperature, and also extended the theory to make quantitative predictions of glass properties.
“Because our theory is based on the counting of bonds and atoms, it’s something that can be solved with pencil and paper,” Mauro said. “We can make accurate predictions of such properties as glass hardness, elastic modulus, viscosity and the glass transition temperature.”
Although their theory, formulated in 2008, has been applied successfully to many oxide glass systems and used in the formulation of industrial glass compositions, this paper is the first time it has been applied to the ZIF metal-organic glass system.
Thomas Bennett at Cambridge University is leading the experimental portion of the work, including the synthesis of ZIF samples, which are currently only millimeters in size.
“There are a lot of challenges that still need to be addressed,” Mauro said. “We hope to use these modeling approaches to predict glasses we can use to ramp up to industrial scale and then commercialize. Wouldn’t it be great to have a glass that is both optically transparent and mechanically ductile?”
Additional authors on the recent paper, titled “Prediction of the Glass Transition Temperatures of Zeolitic Imidazolate Glasses through Topological Constraint Theory,” are Mauro’s Ph.D. students Collin Wilkinson, Kuo-Hao Lee and Karan Doss; Adri van Duin and Yun Kyung Shin, an associate research professor in van Duin’s group, and Thomas D. Bennett, Royal Society University Research Fellow, Cambridge University.
The Institute for CyberScience at Penn State and the Royal Society, UK, funded this work.