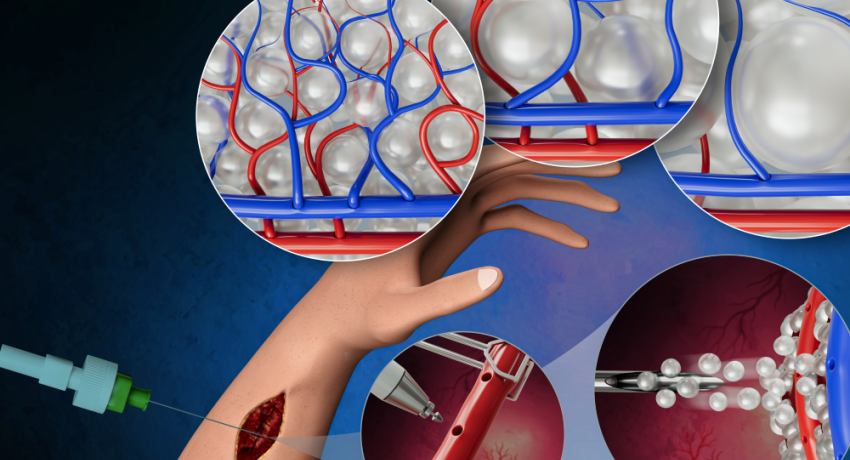
A novel biomaterial combined with a unique microsurgical approach may lead to improved and faster recovery of soft tissue. Credit: Bio-Soft Materials Laboratory/Amir Sheikhi . All Rights Reserved.
By Jamie Oberdick
For soft tissue to recover and regrow, it needs blood vessels to grow to deliver oxygen and nutrients. Sluggish vascularization, however, can slow or even prevent recovery and regrowth of lost or damaged soft tissue after a severe injury or serious illness such as cancer. To speed up the formation and patterning of new blood vessels, Penn State researchers have combined a novel biomaterial with a microsurgical approach used in reconstructive surgery, enabling improved recovery of soft tissue.
According to the team, their work showed via a proof-of-concept seven-day experiment that their technique could accelerate the formation of guided networks of blood vessels. They published their work, which has implications for accelerating vascularization and tissue recovery, in Small.
“Our approach may open opportunities to redefine the tissue vascularization landscape, with widespread applicability in many parts of the human body and for various diseases, including cardiovascular-related ones,” said corresponding author Amir Sheikhi, the Dorothy Foehr Huck and J. Lloyd Huck Early Career Chair in Biomaterials and Regenerative Engineering and assistant professor of chemical engineering with an affiliation with biomedical engineering.
Currently, clinicians commonly use bulk hydrogel scaffolds — support structures made of crosslinked polymer networks — to support blood vessel formation and direction during reconstructive surgery. However, Sheikhi said, the scaffolds are imperfect.
"Bulk hydrogels lack interconnected pores, and the pores they do have are about three orders of magnitude smaller than needed for cell infiltration, or vascularization, requiring degradation and remodeling that may take weeks, if not months,” Sheikhi said. “They have been used as a base for revascularization of tissues, but they yield slow vascularization and random vascular networks.”
This delay can result in various negative outcomes, such as seroma — a buildup of fluid inside the body that may occur after surgery, infection and reconstructive failure. To address this, the researchers produced a two-pronged approach, with Sheikhi partnering with Dino Ravnic, co-corresponding author of the study and Huck Chair in Regenerative Medicine and Surgical Science and associate professor of surgery.
Sheikhi and his lab previously engineered granular hydrogel scaffolds (GHS), which are unique biomaterials made from packed gel particles or microgels. As opposed to bulk hydrogels, which Ravnic noted are commonly used in surgery, GHS enables the blood vessels to regrow in a set pattern. This contrasts with bulk hydrogels, where the blood vessels take on a random appearance as they grow back in bulk hydrogels.
“Not only can we induce blood vessel growth, but then we can pattern it based on the function of the tissue,” Ravnic said. “For example, the skin capillaries are markedly different than the eye capillaries, which are markedly different than the capillaries in the heart, which are different than the ones in the colon. If you want to create a regenerative platform for a particular tissue, you need the capillary bed that allows that to happen, similar to an architectural pattern.”
According to Ravnic, their approach could enable tissue repair and regeneration anywhere in the body.
“Combining the two technologies could be used to promote successful tissue repair across any portion of the body, by either slightly modifying the surgical approach and/or the GHS to apply them where they are needed in the patient,” Ravnic said. “In addition, instead of replacing the damaged or lost tissue with scar tissue, you actually regenerate it.”
Ravnic’s surgery method uses a technique known as micropuncture. This technique involves perforating an existing blood vessel using a tiny needle to aid cells in quickly moving into surrounding tissue. This promotes angiogenic outgrowth, where new blood vessels grow and extend from existing ones. The micropuncture technique prevents the formation of blood clots and significant hemorrhage, common issues in conventional vascular surgery.
Following the micropuncture, the researchers can apply the GHS directly to the wound area where tissue formation is to occur, which serves as tiny building blocks to create a framework to promote blood vessel formation. The GHS features a well-defined void architecture, providing parameters to guide the blood vessels as they grow.
The researchers tested the platform by applying the GHS/microsurgery technique to the hind limbs of rats. They found that blood vessels established around the GHS within seven days, without any observed harmful effects. The researchers also found that using GHS of different microgel sizes, they could control the distances between capillaries in the resulting vessel pattern.
“We strongly believe that this novel platform of GHS and microsurgery for reconstructive surgery and regenerative medicine will help patients grow new blood vessels rapidly,” Sheikhi said.
Sheikhi and Ravnic have affiliations with the Materials Research Institute and the Huck Institutes of the Life Sciences, and Ravnic has an affiliation with the Penn State Cancer Institute. Along with Sheikhi and Ravnic, other authors of the paper from Penn State include Zaman Ataie, Arian Jaberi, Sina Kheirabadi, Angelo Roncalli and Alves E Silva of the Department of Chemical Engineering; Summer Horchler, Srinivas V. Koduru, Jessica C. El-Mallah and Mingjie Sun of the Department of Surgery; and Alexander Kedzierski and Aneesh Risbud of the Department of Biomedical Engineering.
Research reported in this news article and publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R56EB032672. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by Dorothy Foehr Huck and J. Lloyd Huck Early Career Chair, the Penn State College of Engineering’s “Materials Matter at the Human Level” seed grants, the Materials Research Institute’s seed grant for the “Convergent Research at the Intersection of Materials-Life-Health-Environment” and the Huck Institutes of the Life Sciences’ Huck Innovative & Transformational Seed Fund.