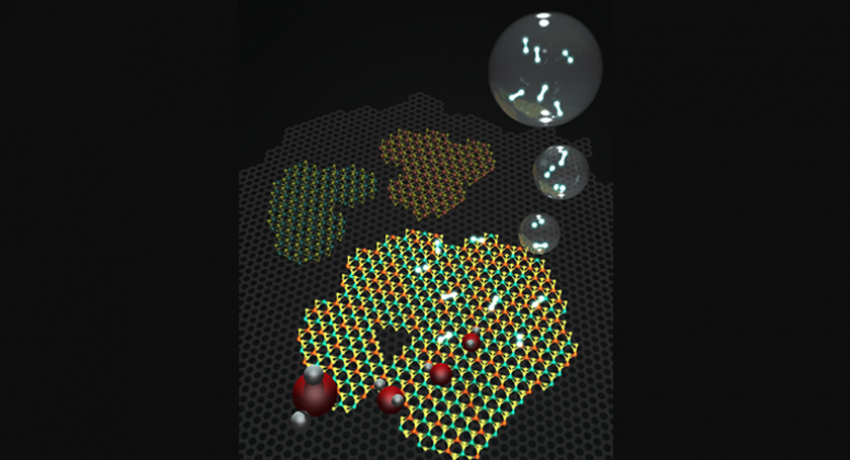
Molecular models representing a 2D heterostructure made of graphene (gray background hexagonal lattice), and islands on top of hexagonal WS2 and MoS, as well as an alloy of the two. Image: Terrones Group
The "clean-energy economy" always seems a few steps away but never quite here. Fossil fuels still power transportation, heating and cooling, and manufacturing, but a team of scientists from Penn State and Florida State University have come one step closer to inexpensive, clean hydrogen fuel with a lower cost and industrially scalable catalyst that produces pure hydrogen through a low-energy water-splitting process.
"Energy is the most important issue of our time, and for energy, fuel cells are crucially important, and then for fuel cells, hydrogen is most important," said Yu Lei, Penn State doctoral student and first author of an ACS Nano paper describing the water-splitting catalyst she and her colleagues theoretically predicted and then synthesized in the lab. "People have been searching for a good catalyst that can efficiently split water into hydrogen and oxygen. During this process, there will be no side products that are not environmentally friendly."
The current industrial method of producing hydrogen — steam reforming of methane — results in the release of carbon dioxide into the atmosphere. Other methods use waste heat, from souces such as advanced nuclear power plants or concentrated solar power, both of which face technical challenges for commercial feasibility. Another industrial process uses platinum as the catalyst to drive the water-splitting process. Although platinum is a near-perfect catalyst, it is also expensive. A cheaper catalyst could make hydrogen a reasonable alternative to fossil fuels in transportation, and power fuel cells for energy storage applications.
"Molybdenum disulfide has been predicted as a possible replacement for platinum, because the Gibbs free energy for hydrogen absorption is close to zero," said Mauricio Terrones, professor of physics, materials science and engineering, and chemistry, Penn State. The lower the Gibbs free energy, the less external energy has to be applied to produce a chemical reaction.
However, experimentally, there are drawbacks to using molybdenum disulfide as a catalyst. In its stable phase, molybdenum disulfide is a semiconductor, which limits its ability to conduct electrons. To get around that problem, the team added reduced graphene oxide, a highly conducting form of carbon. Then, to further decrease the free energy, they alloyed the molybdenum disulfide with tungsten to create a thin film with alternating graphene and tungsten-molybdenum disulfide layers. The addition of tungsten lowers the electrical voltage required to split water by half, from 200 millivolts with pure molybdenum disulfide, to 96 millivolts with the tungsten-molybdenum alloy.
Read the full news story here:
http://news.psu.edu/story/470157/2017/06/01/research/low-cost-scalable-water-...