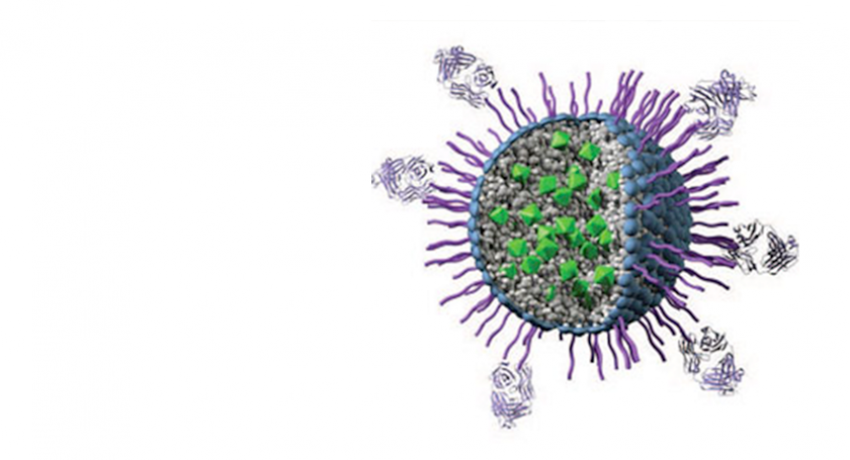
Ceramide nanoliposome, an investigational compound developed by Penn State researchers that targets and destroys cancer while leaving healthy cells unharmed, has been approved for phase one clinical human trials by the U.S. Food and Drug Administration (FDA). Researchers used nanotechnology to stabilize ceramide, a delicate compound that's a known anticancer therapeutic agent. Image: Keystone Nano
An investigational compound developed by Penn State researchers that targets and destroys cancer cells while leaving healthy cells unharmed has been approved for phase one clinical human trials by the U.S. Food and Drug Administration (FDA).
Keystone Nano, a biopharmaceutical company cofounded by James Adair, professor of materials science and engineering, biomedical engineering, and pharmacology, recently was approved to begin clinical trials to assess ceramide nanoliposome for possible use in treating cancer. The trials will seek to establish safe dosing levels and examine the compound’s efficacy as an anticancer therapy. Keystone Nano was founded in 2005 with Mark Kester, former professor of pharmacology at Penn State College of Medicine in Hershey, with the goal of gaining FDA approval for nanoscale biomedical products.
The compound works by weaving ceramide – a known anticancer therapeutic agent that’s never been used in clinical testing to treat cancer – with other fatty lipids that dramatically increase its delicate stability in the body. Upon reaching the tumor, it penetrates the cellular lining before depositing its chemotherapeutic cargo. The coating has resulted in a much greater window of effectiveness over current chemotherapy treatment because ceramide has been found harmless to noncancerous cells in dozens of preclinical animal tests.
A cancer drug’s window of treatment is determined by the gap between the point in which a drug becomes an effective treatment and when it becomes harmful to the patient. Drugs with a larger window of treatment generally pose fewer risks to the patient.
“There’s a whole litany of side effects that cancer patients put up with. About three percent of all patients die from the side effects of the chemotherapy,” said Adair. “We’re very encouraged by ceramide nanoliposomes because the study findings suggest that they could kill cancer while doing little or no harm to the patient.”
Phase one of the trial will recruit patients with solid tumors for testing. If the trial reaches Phase two, it is expected to focus on liver cancer.
Delivering the dose
Kester resolved ceramide’s instability obstacle by protecting the compound in a proprietary fatty coating. Ceramide is then able to freely flow through the body, before eventually being sucked in by the tumor as it funnels metabolic resources from the host.
In dozens of animal tests, the researchers found that the compound remained in the body attacking cancer tumors for more than a day.
Read the full news story here:
http://news.psu.edu/story/473555/2017/06/29/research/investigational-cancer-c...