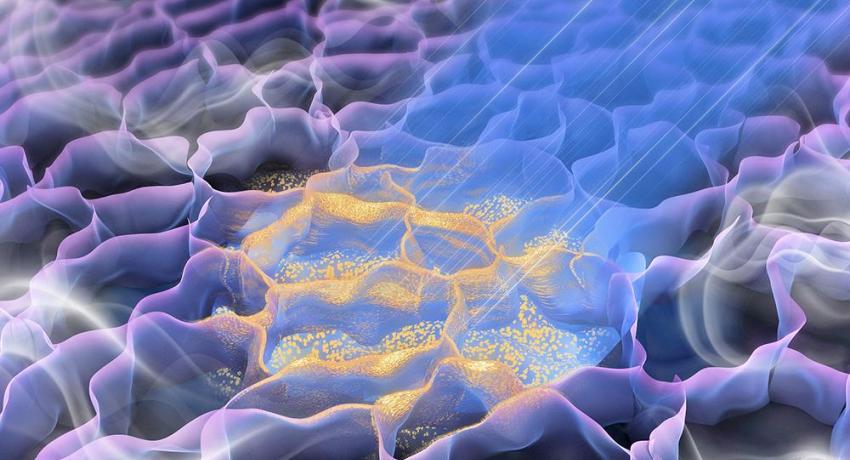
A graphic representation of the laser-induced graphene foam gas sensor during manufacturing. The ray of light represents the laser, which inscribes the nanomaterials onto the graphene foam substrate that will be used to detect gases in the air and in sweat. Credit: Cheng Group. All Rights Reserved.
By Mariah R. Lucas
When used as wearable medical devices, stretchy, flexible gas sensors can identify health conditions or issues by detecting oxygen or carbon dioxide levels in the breath or sweat. They also are useful for monitoring air quality in indoor or outdoor environments by detecting gas, biomolecules and chemicals. But manufacturing the devices, which are created using nanomaterials, can be a challenge.
Penn State researchers recently enhanced their gas sensor manufacturing process through an in situ laser-assisted manufacturing approach, improving on their previous method of drop casting, or dropping materials one by one onto a substrate using a pipette. They published their results in the Chemical Engineering Journal.
“With drop casting, you have to synthesize each part of the sensor separately and then integrate them, which is logistically challenging, takes a long time and is expensive,” said corresponding author Huanyu “Larry” Cheng, James L. Henderson, Jr. Memorial Associate Professor of Engineering Science and Mechanics in the Penn State College of Engineering. “The in situ method allows the materials to be directly synthesized in one place, and the laser speeds up the process.”
In the process, a laser inscribes nanomaterials directly on top of a porous graphene foam substrate. The base material allows for the sensor to be stretchy and flexible when applied on the skin or an object.
According to Cheng, the approach opens opportunities to use different precursors, or nanomaterials, and mix them with different ratios and components. Previously, researchers used graphene oxide and molybdenum disulfide to create the sensors. With the new method, researchers tested four additional classes of materials, including transition metal dichalcogenide, metal oxides, noble metal-doped metal oxides and composite metal oxides.
“A particular nanomaterial allows us to sense different biomarkers or gases, so it’s very important for us to get access to different materials,” Cheng said. “For example, one nanomaterial usually can only detect one target gas molecule. With multiple choices available, you can potentially detect more molecules, improving the sensing capabilities.”
Using several nanomaterials, researchers created an array of several small sensors placed side by side. The capabilities of the array can be compared to a human nose, Cheng said.
“The nose evolved to detect millions of smells using millions of cells,” Cheng said. “In the same way, each of the sensors is able to detect a different chemical or particle.”
With the new sensor design, researchers eliminated the need for a separate heat source, further decreasing the complexity of manufacturing the device. The new design integrates the gas-sensitive nanomaterials on a single line of porous graphene foam, as compared with the old design, where nanomaterials filled the gaps between electrodes. The resistance in the single line of porous graphene foam induces Joule heat for self-heating.
The result is a sophisticated sensor that has several applications, including monitoring and alerting the user of a quick uptick in gases, such as on an industrial site, or an accumulation of gases over time, such as in the case of pollution.
In the future, researchers plan to improve the sensor’s capabilities by programming nanomaterial composites to target specific gases or to identify multiple gas species in complex mixtures.
In addition to Cheng, the co-authors include Jiang Zhao, Xiaohong Ding, Shangbin Liu, Jia Zhu, and Yuyan Gao, Penn State Department of Engineering Science and Mechanics; Ning Yi, Penn State Department of Materials Science and Engineering; Alexander C. Castonguay, Penn State Department of Chemistry; and Lauren D. Zarzar, Penn State Department of Chemistry and Department of Materials Science and Engineering.
The National Institutes of Health, the National Science Foundation and Penn State supported this work.